Esters are an essential class of organic compounds with a wide range of applications in our daily lives, from the fragrances we enjoy to the food we eat. Understanding esters is crucial not only for those in the fields of chemistry and biology but also for anyone interested in the science behind everyday products. In this article, we will explore the definition, structure, properties, synthesis, and applications of esters in a detailed yet easily comprehensible manner. By the end, you’ll have a comprehensive understanding of what esters are and why they are so important.
1. What is an Ester?
1.1 Definition of Ester
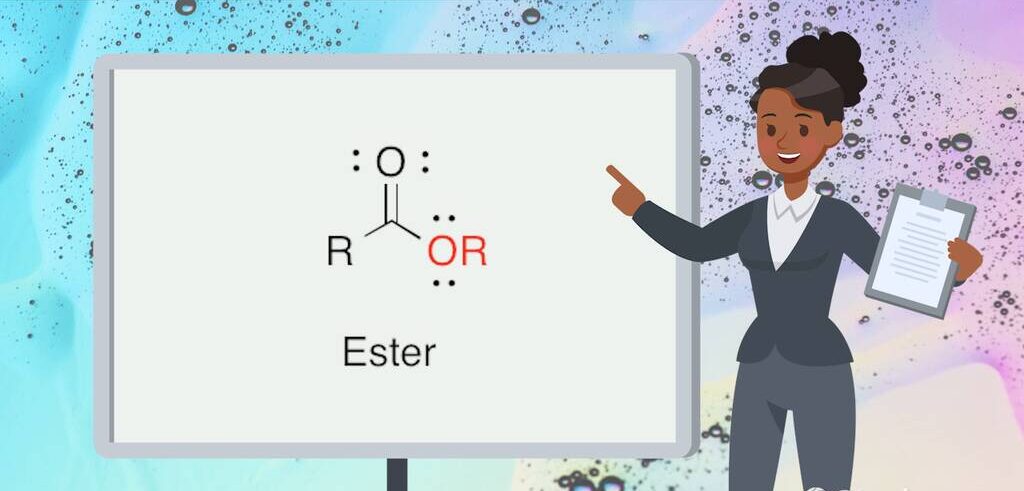
An ester is an organic compound that results from the reaction between an acid and an alcohol. This reaction, known as esterification, typically involves a carboxylic acid and an alcohol, leading to the formation of an ester and water. The general chemical formula for an ester is RCOOR’, where R and R’ represent alkyl or aryl groups. The “R” groups can vary, leading to a wide diversity of esters with different properties and uses.
1.2 The Structure of Esters
The structure of an ester includes a carbonyl group (C=O) bonded to an oxygen atom, which is in turn bonded to an alkyl or aryl group. This structural arrangement gives esters their characteristic properties. The carbonyl group is crucial because it influences the reactivity and polarity of the ester, making it an essential functional group in organic chemistry.
2. How Esters Are Formed: The Esterification Process
2.1 The Basics of Esterification
Esterification is the chemical reaction in which an acid reacts with an alcohol to form an ester and water. This process is typically catalyzed by an acid, such as sulfuric acid, which accelerates the reaction. The reaction is reversible, meaning that under different conditions, the ester can be hydrolyzed back into the original acid and alcohol.
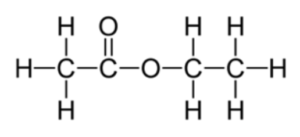
2.2 Detailed Mechanism of Esterification
The esterification process begins with the protonation of the carbonyl group of the carboxylic acid, making it more electrophilic and thus more susceptible to attack by the nucleophilic alcohol. The alcohol then attacks the carbonyl carbon, forming a tetrahedral intermediate. This intermediate eventually loses a molecule of water, leading to the formation of the ester.
2.3 Factors Influencing Esterification
Several factors can affect the esterification process, including the concentration of reactants, temperature, and the presence of a catalyst. Increasing the concentration of either the acid or alcohol can drive the reaction forward, while higher temperatures generally increase the reaction rate. The use of a strong acid catalyst is also crucial for efficient esterification.
3. Properties of Esters
3.1 Physical Properties
Esters are typically characterized by their pleasant, often fruity odors, which make them popular in the fragrance and flavor industries. They are generally colorless liquids at room temperature, though some can be solid. Esters have relatively low boiling points compared to acids or alcohols of similar molecular weight due to the absence of hydrogen bonding between ester molecules.
3.2 Chemical Properties
Chemically, esters are relatively stable but can be hydrolyzed back into their parent acid and alcohol under acidic or basic conditions. This hydrolysis is the reverse of the esterification reaction. Esters can also participate in other chemical reactions, such as transesterification, where an ester reacts with an alcohol to form a different ester.
3.3 Solubility
The solubility of esters in water depends on the size of the alkyl groups attached to the ester. Small esters are somewhat soluble in water due to their ability to form hydrogen bonds with water molecules. However, as the alkyl group size increases, the solubility decreases because the hydrophobic nature of the alkyl group dominates.
4. Types of Esters
4.1 Simple Esters
Simple esters, such as ethyl acetate and methyl formate, are among the most commonly used esters. They are usually formed from simple carboxylic acids and alcohols and are widely used as solvents, flavors, and fragrances.
4.2 Complex Esters
Complex esters, such as triglycerides, are formed from more complicated alcohols and acids. Triglycerides, for example, are esters of glycerol and fatty acids and are a major component of natural fats and oils. These esters play a critical role in biology, serving as energy storage molecules.
4.3 Polyesters
Polyesters are a class of polymers formed from ester monomers. They are used extensively in the production of fabrics and plastics. Polyethylene terephthalate (PET), used in plastic bottles and synthetic fibers, is one of the most well-known polyesters.
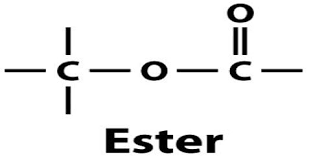
5. Applications of Esters
5.1 Esters in Food and Flavors
Esters are widely used in the food industry as flavoring agents. Their pleasant, fruity odors make them ideal for adding flavor to candies, ice creams, and beverages. For example, isoamyl acetate is known for its banana flavor, while ethyl butyrate has a pineapple-like taste.
5.2 Esters in Fragrances
The fragrance industry heavily relies on esters due to their aromatic properties. Many natural and synthetic esters are used in perfumes, colognes, and scented products. Esters like ethyl acetate and linalyl acetate are common in the formulation of floral and fruity fragrances.
5.3 Esters as Solvents
Esters are also widely used as solvents in industries such as paints, coatings, and adhesives. Their ability to dissolve a wide range of substances makes them valuable in manufacturing and industrial processes. For instance, ethyl acetate is a common solvent in nail polish removers and paint thinners.
5.4 Esters in Medicine
In the pharmaceutical industry, esters are used in the formulation of various drugs. Some esters are used as prodrugs, which are inactive compounds that metabolize into active drugs in the body. For example, aspirin (acetylsalicylic acid) is an ester that, when ingested, is hydrolyzed to salicylic acid, the active compound.
5.5 Biological Importance of Esters
Esters play a critical role in biology, particularly in the formation of fats and oils, which are crucial for storing energy in living organisms. Phospholipids, which form the structural basis of cell membranes, are also esters. Additionally, esters are involved in the transport of fatty acids in the body.
6. Environmental Impact of Esters
6.1 Biodegradability of Esters
Many esters are biodegradable, meaning they can be broken down by microorganisms into harmless substances. This property makes them environmentally friendly compared to some other organic compounds. However, the rate of biodegradation can vary depending on the ester’s structure.
6.2 Esters in Pollution
Some esters, particularly those used as solvents, can contribute to environmental pollution if not properly managed. Volatile esters can evaporate and contribute to air pollution, while others may contaminate water sources. It’s crucial to handle and dispose of ester-containing products responsibly to minimize their environmental impact.
6.3 Sustainable Alternatives
In response to environmental concerns, researchers are developing sustainable alternatives to traditional esters. Bio-based esters, derived from renewable resources, are gaining popularity as eco-friendly options. These esters are produced from plant oils and other natural sources, reducing reliance on fossil fuels and lowering carbon footprints.
7. Synthesis of Esters
7.1 Laboratory Synthesis of Esters
In a laboratory setting, esters can be synthesized through various methods, with esterification being the most common. Other methods include the use of acid chlorides, anhydrides, and transesterification reactions. Each method has its advantages and is chosen based on the desired ester and the available starting materials.
7.2 Industrial Synthesis of Esters
On an industrial scale, esters are synthesized in large quantities for use in various applications. Industrial processes often involve continuous esterification or transesterification reactions, optimized for efficiency and yield. These processes are designed to produce high-purity esters for use in food, pharmaceuticals, and other industries.
7.3 Catalysts in Ester Synthesis
Catalysts play a crucial role in the synthesis of esters, both in laboratory and industrial settings. Acid catalysts, such as sulfuric acid or p-toluenesulfonic acid, are commonly used to accelerate esterification reactions. In some cases, enzymatic catalysts are employed, especially in the production of bio-based esters.
8. Esters in Everyday Life
8.1 Esters in Household Products
Many household products contain esters due to their pleasant scents and solvent properties. Esters are found in cleaning agents, air fresheners, and personal care products like lotions and shampoos. Their versatility makes them a common ingredient in a wide range of everyday items.
8.2 Esters in the Food Industry
In addition to flavoring agents, esters are used as food additives and preservatives. Some esters are added to extend the shelf life of products or to stabilize certain food ingredients. For instance, mono- and diglycerides, which are esters, are used as emulsifiers in processed foods.
8.3 Esters in the Textile Industry
The textile industry heavily relies on esters, particularly polyesters, for the production of fabrics. Polyester fibers are used to make clothing, upholstery, and other textiles due to their durability and resistance to shrinking and wrinkling. The versatility of esters makes them indispensable in textile manufacturing.
9. Future Trends in Ester Research
9.1 Advances in Green Chemistry
As environmental concerns continue to grow, research into green chemistry is leading to new methods for synthesizing esters. These methods focus on reducing waste, using renewable resources, and minimizing the environmental impact of ester production. Future trends may include the development of biodegradable and bio-based esters that offer the same benefits as traditional esters without the ecological drawbacks.
9.2 Esters in Biotechnology
Esters are also becoming increasingly important in biotechnology, particularly in the development of biofuels and bioplastics. Esters derived from natural sources are being explored as potential alternatives to fossil fuel-based products. This research holds promise for reducing our dependence on non-renewable resources and creating more sustainable industries.
9.3 Personalized Medicine
In the field of medicine, esters may play a role in the development of personalized treatments. By modifying ester structures, researchers can create tailored drug delivery systems that improve the efficacy and safety of medications. This approach could lead to more effective treatments with fewer side effects.
10. Conclusion
Esters are a fascinating and versatile class of organic compounds with a wide range of applications in our daily lives. From the pleasant scents in perfumes to the essential roles in biological processes, esters are everywhere. Understanding their chemistry, properties, and uses can enhance our appreciation of the science behind many everyday products